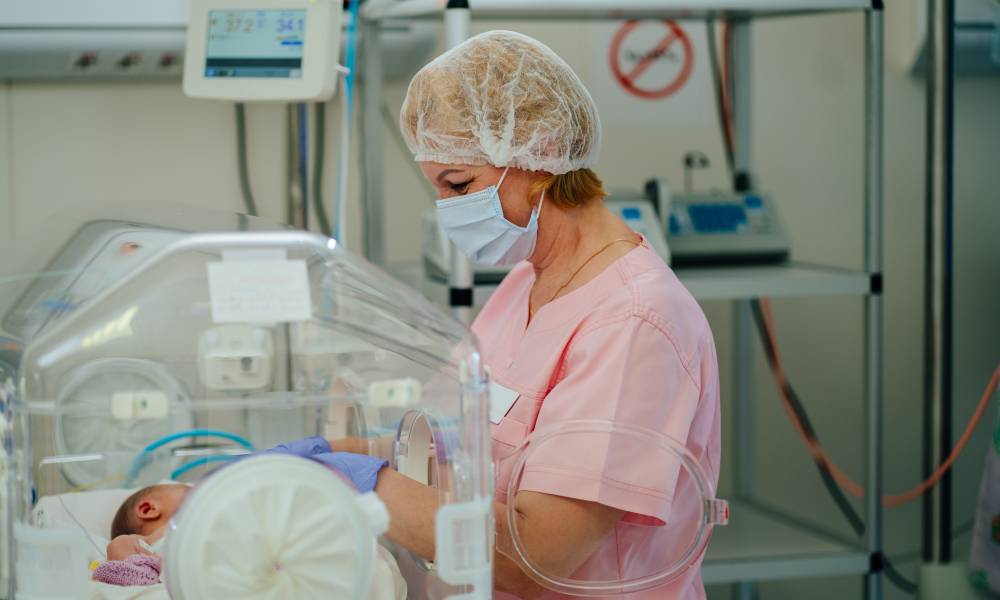
While conversations about patient safety often center on medication errors, staffing shortages or clinical protocols, one often overlooked patient safety factor is the infection risk tied to improperly cleaned medical equipment. The risks are especially high for the most vulnerable patients in the healthcare system: newborns in the Neonatal intensive care unit (NICU).
Vulnerability and Vigilance
One in every 10 babies in the U.S. is born prematurely and spends their first fragile weeks in the NICU. These specialized, high-tech environments are designed to stabilize premature or critically ill newborns, giving them a fighting chance. However, the same environment that is designed to sustain and strengthen life can also introduce risks.
In 2016 the Joint Commission reported that 74% of all immediate threat-to-life declarations were tied to improperly sterilized or disinfected equipment, including neonatal devices. This underscores the urgency of infection prevention in pediatric and neonatal care. Sterilization and disinfection are not optional best practices; they are lifesaving measures that must be carried out with the same intensity as clinical care itself.
NICU equipment needs to be cleaned and sterilized thoroughly after every use. For example, take isolettes (also known as incubators, warmers or Giraffes) – more than 60 minutes is required to disassemble, clean properly, dry and then reassemble each unit, a process that must be repeated at least once a week. In a level 3 NICU plus woman/baby center with more than 65 devices, that’s roughly 65 hours a week or 3,400 hours annually.
This time demand places extraordinary pressure on environmental services (EVS) teams, who must balance speed with precision, and on nurses, who assume cleaning responsibilities when staffing is stretched thin. Risks are amplified by frequent use, complex disassembly and multiple caregivers handling the same devices throughout the day. In such high-contact environments, even minor lapses in care station cleaning can create hidden reservoirs for pathogens to thrive.
Structured Equipment Management Programs
As hospitals and healthcare systems face staffing shortages and budget constraints, engaging a third-party partner is vital to help establish and execute an equipment management program focused on cleaning in the NICU. These partners play a pivotal role by standardizing processes and providing specialized expertise, alleviating the burden on clinical staff and enabling them to spend more time with patients.
It is the responsibility of healthcare professionals and facilities to ensure that infection prevention and sterilization routines follow OEM standards are carried out with the same intensity as clinical care itself. A third-party partner is crucial to ensure that strict adherence to protocols is consistently followed, minimizing the chance that any component is overlooked. These programs focus on efficiency, quality and reducing risk for healthcare providers and patients alike.
Organizational strategies provided by third-party partners can include:
- Standardizing protocols so caregivers follow the same clear steps, minimizing skipped processes under pressure. Cleaning and sterilization should follow manufacturer-recommended (OEM) guidelines, with visual indicators helping confirm it is patient ready.
- Staffing and training EVS and nursing teams to make sure they have the time needed for thorough equipment cleaning. Dedicated roles for cleaning and managing equipment maintain consistency and safety.
Protection Through Prevention
Across the nation’s 1,424 NICUs, hospital-acquired infections (HAIs) remain a serious threat, with studies showing that nearly 9% of infants had late-onset sepsis, a major cause of morbidity and mortality. Because hospital equipment is a significant part of caring for the most fragile patient population, each piece of equipment in a NICU holds the potential to either protect or harm.
For families, the consequences are devastating. Infections can prolong hospital stays or increase the risk of long-term complications such as neurological impairment or chronic lung disease.
Protecting vulnerable infants requires individuals to foster a culture of vigilance and adherence to proven clinical best practices, such as:
- Hand hygiene is the first and most reliable defense. Even the smallest lapse can have serious consequences. Ongoing reminders and built-in workflow prompts can aid staff in staying consistent.
- Clear distinction between cleaning, disinfection and sterilization, ensuring each step is performed correctly and to OEM standards.
- Proper sterilization of equipment, including feeding tools, IV lines and ventilator tubing.
- Comprehensive environmental cleaning and safe handling of all surfaces and devices, as pathogens can survive for days on plastics, metals and fabrics.
- Safe storage and handling of sterile supplies, ensuring integrity is maintained through proper wrapping, storage conditions and handling.
- Continuous staff education and monitoring to reinforce accountability and close gaps. Implementing real-time feedback or peer monitoring can help improve compliance.
While these practices appear to be straightforward, they are challenging to consistently implement in a fast-paced NICU environment, where staff balance heavy caseloads, complex equipment and limited time. That is why having a trusted third-party partner to implement and support these best practices is extremely valuable, as their expertise ensures equipment is thoroughly cleaned and ready for every patient use.
A Collective Responsibility
Premature infants depend not just on medical advancements but also on the diligence of healthcare professionals who shape their environment. In neonatal care, infection prevention is a shared responsibility that involves clinical teams, hospital staff, biomedical engineers and partners specialized in equipment cleaning and management.
Ultimately, vigilance is the cornerstone of safety. Every protocol followed without compromise and every piece of equipment meticulously sterilized represents more than items on a checklist. With the support of trusted third-party partners, hospitals can extend that vigilance beyond their internal teams, ensuring infection prevention is consistent, comprehensive and sustainable. Together, these practices form the framework that protects the smallest and most fragile patients.

Andrew Yamarick
Andrew Yamarick is the Business Unit General Manager of Medical Technology Management at Agiliti, where he oversees programs that help healthcare providers maximize equipment utilization, lifecycle, and financial performance. Under his leadership, the MTM team develops data-driven optimization strategies that reduce costs and ensures hospitals have the right equipment available when and where it’s needed to improve operational efficiency and care. Before joining Agiliti, he spent time at Press Ganey and nearly 11 years with GE Healthcare in a variety of commercial and operational leadership roles. Andrew graduated from The Pennsylvania State University and has an MBA from the Katz Graduate School of Business at the University of Pittsburgh.